Description
SafetyTector™ S: Protect the User, Detect the Analyte
SafetyTector™ S is an extraction and dilution buffer that inactivates COVID-19 in saliva specimens and nasopharyngeal swabs.
By inactivating COVID-19 in samples, SafetyTector™ S eliminates the high risk of infection from the specimen or other test materials during and after testing. This safety measure is essential for in vitro diagnostic medical devices. The inactivation ability of SafetyTector™ S provides user safety that has not been achieved by other virus-inactivating extraction buffers.
Swabs can be extracted directly with SafetyTector™ S. Samples must be diluted in SafetyTector™ S at least 1:4. At this dilution, SafetyTector™ S inactivates COVID-19 within 1 minute, making the samples no longer infectious. Virus inactivation was confirmed and tested with infectious viruses in a Biosafety Level 3 laboratory.
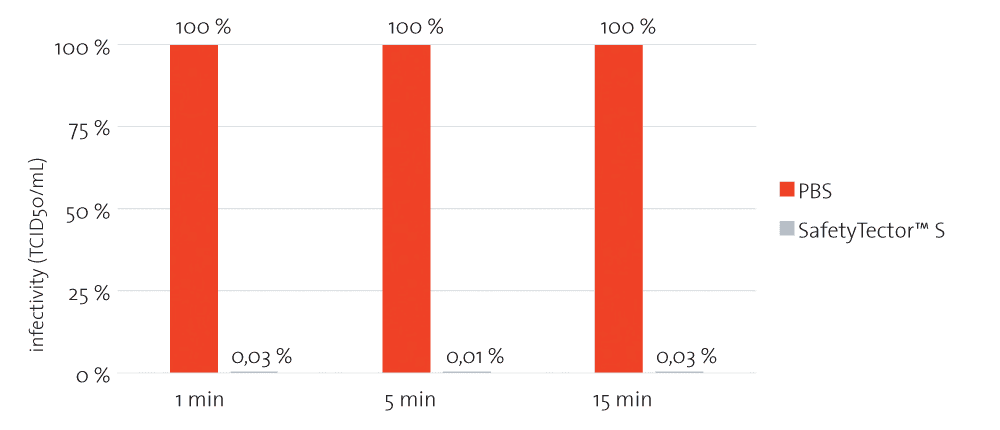
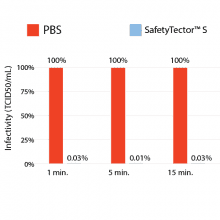
Figure 1: Saliva samples spiked with COVID-19 were mixed 1:4 with PBS or SafetyTector™ S and incubated for the indicated times at room temperature. After incubation, samples were titrated and added on Vero E6 cells. After 5 to 7 days, infectivity was determined according to the Reed-Muench-method. After only 1 min of incubation with SafetyTector™ S, no remaining infectivity was detectable with this assay.
Samples diluted in SafetyTector™ S can be used directly in lateral flow assays for rapid antigen tests. In lateral flow assays, SafetyTector™ S replaces the dilution buffer, extraction buffer, chase buffer or flow buffer. SafetyTector™ S can also be used in other immunoassays such as ELISA, protein arrays and bead-based assays.
- Inactivates virus in COVID-19 rapid antigen tests while keeping the antigens detectable
- Provides user safety unmatched by other commercial buffers
- Well suited for rapid lateral flow assays
- Inactivation occurs in 1 minute
- Tested in a Biosafety Level 3 laboratory
- Prevents the need for treating the waste products of testing as infectious biohazards
- COVID-19 inactivation in saliva and mucosal swab samples in Lateral Flow Assays
- Can also be used in other assays such as ELISA, Protein Arrays, Bead-based Assays and Immuno-PCR
- User safety in rapid antigen tests for COVID-19
- 400 902-1 - SafetyTector™ S, OEM, 1L
- 400 902-5 - SafetyTector™ S, OEM, 5L
- 400 902-10 - SafetyTector™ S, OEM, 10L
Contact us for pricing for larger volumes.



4_220x220p19sf.png)